Synthetic Iron-Sulfur Clusters
Replicating Enzymatic Electron and Proton Transfer Mechanisms
Iron-sulfur clusters are essential cofactors for life and are ubiquitous in most enzymatic systems where they participate in electron transfer chains, coupled-proton electron transfers or directly catalyze complex transformations. Based on the design and synthesis of new iron-sulfur clusters, we aim to provide functional models for these transformations.
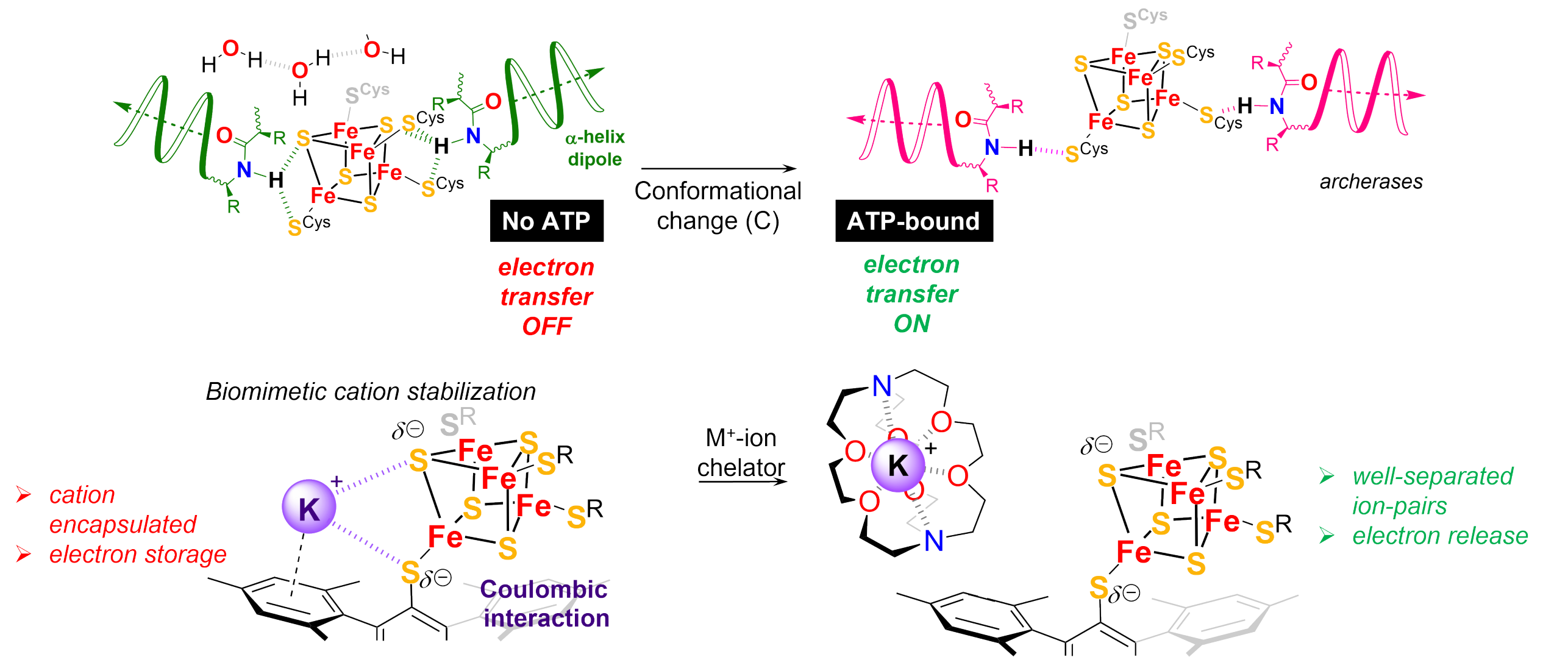
We have in particular demonstrated that synthetic Fe4S4 clusters can act as concerted proton electron transfer (CPET) mediators for electrocatalytic generation of metal hydride species, in analogy with the mechanisms observed in the Fe-Fe hydrogenase enzyme and exploited this feature in the context of CO2 reduction to formic acid. Further exploring bio-inspired strategies for electron transfers and storage, we reported the preparation of the first complete redox series of Fe4S4 complexes that covers all accessible oxidation states, including the elusive all-ferrous oxidation state. Replicating the modulation of electric fields observed in gating enzymatic mechanism, we demonstrated that Fe4S4(SR)4 models can exhibit dynamic redox potentials on-demand.
Selected Publications:
external page Gated electron transfers at synthetic iron-sulfur cubanes
L. Grunwald, M. Inoue, P. Cendoya Carril, M. Wörle, V. Mougel*
Chem, 2024, 10(1), 365-387
external page A complete biomimetic iron-sulfur cubane redox series
L. Grunwald, M. Clémancey, D. Klose, L. Dubois, S. Gambarelli, G. Jeschke, M. Wörle, G. Blondin, V. Mougel*
PNAS, 2022, 119 (31), e2122677119
external page Electrocatalytic metal hydride generation using CPET mediators
S. Dey, F. Masero, E. Brack, M. Fontecave, V. Mougel*
Nature, 2022, 607 (7919), 499-506